Infectious salmon anemia
Infectious salmon anemia (ISA) is a serious, contagious viral disease affecting Atlantic salmon. The ISA virus has also been detected in farmed rainbow trout and wild sea trout.
ISA is notifiable both in Norway and the EU (Categories C+D+E), and infection with the ISA virus is also listed by the World Organization for Animal Health (WOAH). Suspected cases of ISA must be reported immediately to the Norwegian Food Safety Authority that publishes notifications of suspected and confirmed outbreaks on its website, and reports detections of pathogenic ISA virus to the European Commission and WOAH. Both suspected and confirmed outbreaks of ISA can affect the export of Atlantic salmon and rainbow trout.
Pathogen and Transmission
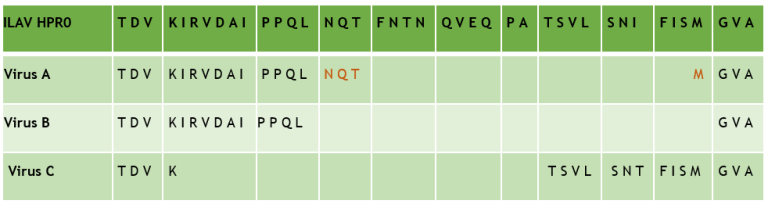
The virus that causes infectious salmon anemia (ISA) is an aquatic orthomyxovirus structurally similar to the influenza virus. A distinction is made between non-pathogenic ISA virus (ISAV HPR0) and pathogenic ISA virus (ISAV HPR∆). ISAV HPR∆ evolves from ISAV HPR0 through a process involving changes in two surface proteins. Deletion of parts of the viral genome coding for amino acids in the hypervariable region (HPR) of the virus’s hemagglutinin-esterase, along with modifications near the cleavage site of the fusion protein, appear together to enhance the virus’s ability to penetrate host cells. However, mortality is influenced not only by the virus's properties, but also by host resistance and environmental factors.
The virus initially establishes itself in the epithelial layer covering the fish’s outer surfaces, such as the mucosal tissues of the gills and skin and subsequently infects the endothelial cells lining the interior of blood vessels and the heart. Typically, an outbreak begins in a single net pen and spreads to adjacent pens over the course of weeks to months. ISA can often be characterized as a "smoldering fire," with low mortality, although instances of very high mortality have also been reported. While vertical transmission of ISA virus from broodfish to offspring cannot be entirely ruled out, it likely plays a minor role in the spread of ISA in Norwegian aquaculture.
Epidemiological data suggest that a proportion of ISAV HPR0 infections result in the development of ISAV HPR∆. The emergence of ISAV HPR∆ from ISAV HPR0 is considered the most probable explanation for isolated outbreaks. It has been documented that such transitions can occur in the field and that isolated ISA outbreaks may be linked to inadequate biosecurity practices, such as a lack of separation between generations and stress. However, there is insufficient knowledge regarding the actual risk that ISAV HPR0 poses for the development of ISAV HPR∆, including whether species other than Atlantic salmon may serve as reservoirs, how frequently ISAV HPR0 transitions to ISAV HPR∆, and what factors drive this development.
Clinical Signs
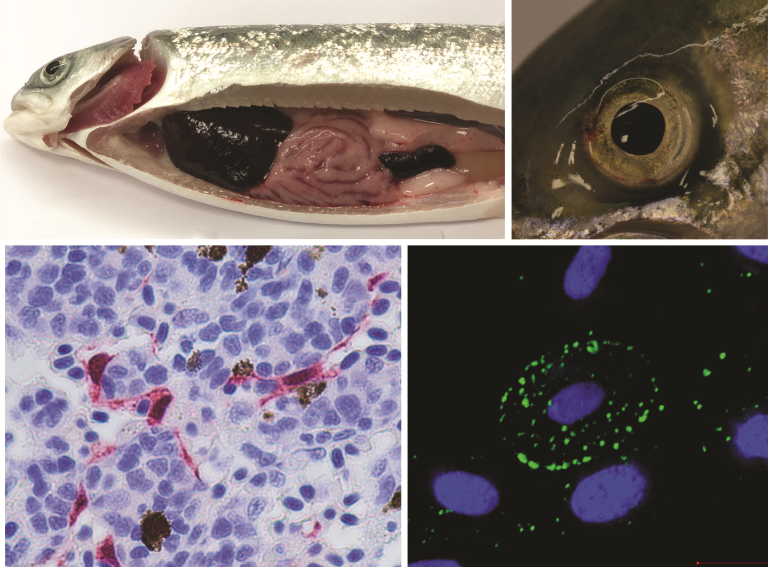
At necropsy, signs of circulatory problems are usually observed, along with varying degrees of hemorrhaging in the skin and internal organs. The liver may appear dark, and the kidneys or spleen may be swollen, with blood pooling in the intestines. The daily mortality in pens with sick fish is typically relatively low, around 0.5–1‰.
ISAV HPR0 is not associated with disease symptoms or organ changes (pathology).
Diagnosis
Personnel from the fish health service or the Norwegian Food Safety Authority perform necropsies on fish and collect relevant samples in the field. The laboratory receives a description of the disease history and visible changes, along with tissue samples from various organs. Both in cases of suspected ISA and for surveillance purposes, relevant samples are typically first analyzed using real-time RT-PCR. If the virus is detected, the hyper polymorphic region (HPR) is sequenced to determine whether it is ISAV HPR0 or ISAV HPR∆. Formalin-fixed tissue is examined using histopathological techniques to identify changes characteristic of ISA, and the virus can be detected through immunohistochemistry (staining virus in tissue sections) and cell culture. It is required that the virus is detected using two different methods to establish an ISA diagnosis.
A larger portion of the virus's genome, beyond just the HPR region, is sequenced from a sample of specimens from all locations where the virus is detected, provided there is sufficient viral material in the samples. This allows for the assessment of genetic relatedness between viruses detected at different locations, which is essential for tracing the spread of the disease. In addition to evaluating the genetic relatedness of viruses, deletions in the HPR region are important for assessing whether transmission between two locations where closely related or identical viruses are found is likely. Viruses with the same or compatible deletions are consistent with transmission between locations, while viruses with different, incompatible deletions argue against transmission between locations, even if the viruses are closely related, and instead suggest a common origin such as ISAV HPR0 circulating in the sea or being present at the hatchery.
The Norwegian Veterinary Institute is the national reference laboratory for fish diseases and the reference laboratory for ISA virus infection for the World Organization for Animal Health (WOAH). In line with the institute's obligations as a reference laboratory, quality-controlled virus sequences for segments 5 and 6 detected during outbreaks, suspicions, and surveillance are published in a public international database (GenBank). The sequences are named based on their geographic origin, year of detection, and the journal number at the Norwegian Veterinary Institute. Additionally, information such as location number, location name, sampling date, and species is reported.
Differential diagnoses
Several disease conditions may be confused with ISA during initial diagnostic investigations, especially in cases where anemia, circulatory system disturbances, and hemorrhages are present. There are several documented instances of ISA where the initial suspicion was CMS (Cardiac Myopathy Syndrome), and ISA has also been linked to issues with winter ulcers and systemic infections with Moritella viscosa.
Surveillance
From 2019 to 2024, the Norwegian Veterinary Institute conducted investigations as part of the monitoring program for ISAV HPR0 in Norwegian hatcheries, commissioned by the Norwegian Food Safety Authority. Ninety fish from each hatchery were sampled every other year, and ISAV HPR0 was detected in at least one sample from approximately one in ten hatcheries each year. The true prevalence is likely higher, as infection with ISAV HPR0 is transient. On the other hand, there are several examples where ISAV HPR0 has been detected repeatedly at the same hatchery.
Learn more about monitoring of ISAV HPR0 in hatcheries.
All facilities located within ISA restriction zones must conduct monthly health visits and sampling to detect ISA as early as possible. The samples are analyzed either at the Norwegian Veterinary Institute or by private laboratories. The Norwegian Veterinary Institute compiles the results for the Norwegian Food Safety Authority.
Learn more about surveillance of pathogenic infectious salmon anemia virus (ISAV HPR∆) in restricted zones in Norway in 2022.
Occurence
The first outbreak of infectious salmon anemia (ISA) was detected in Norway in 1984 and has since only been reported in farmed Atlantic salmon. The number of ISA outbreaks peaked in 1990 with 80 outbreaks. In the late 1980s and early 1990s, Norway implemented a series of measures to combat the disease. Since 1993, the number of outbreaks has varied between one and 25 per year.
In the last decade, ISA has been confirmed in two hatcheries. Both had supplied smolt to several grow-out farms where ISA was also detected. In both cases, the ISA virus at the hatcheries was either identical or closely related to the virus found at the affected grow-out farms. During the same period, ISAV HPR0 was detected in about ten hatcheries that supplied smolt to one or more farms with ISA outbreaks. Again, the ISA viruses causing the outbreaks were identical or closely related to ISAV HPR0 found at the hatchery from which the fish originated.
Pathogenic virus has also been detected in rainbow trout in connection with ISA outbreaks in salmon, though no disease has been reported in this species. The role of rainbow trout infection in the spread of the virus is unknown, but it has been shown that the infection in rainbow trout can persist for several months.
ISA outbreaks have been reported from most countries with commercial salmon farming, including Scotland, the Faroe Islands, Chile, Canada, and the USA.
Control Measures
ISA outbreaks are met with strict measures. Upon confirmation of ISA, the Norwegian Food Safety Authority issues a regulation establishing a restricted zone. The restricted zone includes one zone for disease control (the protection zone) and one for surveillance (the surveillance zone), with detailed guidelines regarding operational conditions, intensified disease monitoring, and sampling.
Usually, the Norwegian Food Safety Authority requires that diseased fish be destroyed or slaughtered as quickly as possible. All fish in a facility with an ISA diagnosis are considered infected, but the Norwegian Food Safety Authority determines on a case-by-case basis how long clinically healthy fish may remain in the facility. The risk of virus spread to neighboring farms is an important factor in the Norwegian Food Safety Authority’s assessment.
The goal of these measures is to combat the viral infection and limit its spread. Vaccines for ISA have been developed, and vaccination has become increasingly common in recent years. In 2024, nearly 200 million fish were vaccinated in Norway.
Reports
- Fiskehelserapporten 2023
- Infeksiøs lakseanemi (ILA) - utbrudd og statistikk
- Biosikkerhetstiltak mot ILA i settefisk (ILA-SAFE)
- Utbredelse og betydning av ILA-virus i den norske oppdrettspopulasjonen av laksefisk
- Smittemodell for ILAV HPR0
- Betydning av ILAV HPR0 for utbrudd av ILA
